
Precision Medical PCB Assembly: Ensuring Safety and Reliability in 2025
When it comes to medical devices, nothing is more important than safety and reliability. The printed circuit boards, or PCBs, inside these machines are like the brains, and getting them right is super important. This article talks about how medical PCB assembly is done with a focus on making sure everything works perfectly, especially as we head into 2025. We’ll look at what makes medical PCBs special, the tech used, how they’re tested, and what rules they have to follow. Plus, we’ll peek at what’s coming next in this field.
Table of Contents
Key Takeaways
- Medical PCB assembly needs to be incredibly precise because patient safety is on the line. This means using the right manufacturing methods and really strict quality checks.
- Advanced techniques like SMT, THT, and HDI are used to make PCBs smaller, more complex, and more reliable for all sorts of medical gadgets.
- Testing is a big deal. Things like AOI, X-ray, and functional tests are done over and over to catch any problems before the devices are used.
- Following rules from places like the FDA and ISO is not optional. Manufacturers have to keep detailed records and make sure their processes are approved.
- The future is looking at flexible PCBs for wearables and smart tech like IoT and AI to make medical devices even better and more connected.
Precision Medical PCB Assembly: Ensuring Safety and Reliability
When it comes to medical devices, there’s really no room for error. The printed circuit boards, or PCBs, inside these devices are like the brains, controlling everything from a simple monitor to complex surgical equipment. That’s why precision in their assembly is so important. It’s not just about making them work; it’s about making them work perfectly, every single time, to keep patients safe.
Understanding the Critical Role of Medical PCB Assembly
Medical PCBs are the backbone of countless healthcare technologies. Think about pacemakers, diagnostic imaging machines, or even simple blood glucose meters. Each one relies on a meticulously assembled PCB to function correctly. The assembly process itself involves placing tiny electronic components onto a board, connecting them in a specific way to perform a function. For medical applications, this process needs to be incredibly accurate. Even a slight misplacement or a faulty connection can have serious consequences. We’re talking about devices that directly impact patient health, so the stakes are incredibly high. This is why manufacturers focus so much on getting the assembly just right, using specialized techniques and strict oversight.
Key Considerations for Medical PCB Assembly
There are a few big things to keep in mind when assembling PCBs for medical use. First off, the components themselves need to be top-notch. Not all electronic parts are created equal, and medical devices require components that are reliable and built to last. Then there’s the actual assembly process.
We often see a mix of Surface Mount Technology (SMT) and Through-Hole Technology (THT). SMT is great for fitting lots of small parts into tight spaces, which is common in modern, smaller devices. THT, on the other hand, is good for larger, more robust parts that need a strong connection, like connectors. The soldering process also needs careful control, with temperatures managed precisely to avoid damaging sensitive parts. It’s a delicate balance.
Here’s a quick look at some common assembly methods:
- SMT Assembly: Uses automated machines to place tiny components directly onto the board’s surface. It’s fast and precise, ideal for dense designs.
- THT Assembly: Involves inserting component leads through holes in the PCB and soldering them on the other side. This provides a mechanically stronger connection.
- Mixed Technology: Combining both SMT and THT on the same board to take advantage of each method’s strengths.
Partnering for Excellence in Medical PCB Assembly
Because medical PCB assembly is so complex and critical, most companies don’t try to do it all in-house. They partner with specialized assembly providers. These partners have the advanced equipment, the trained staff, and the quality systems in place to handle the demanding requirements of the medical industry. They can manage everything from sourcing the right components to the final testing, making the whole process smoother and more reliable. Finding the right partner is key to getting high-quality, safe medical devices to market. It’s about building trust and working with folks who understand the unique challenges of healthcare electronics, like those at ANZER Electronics.
The entire process, from the initial design to the final product, needs to be documented thoroughly. This isn’t just good practice; it’s often a regulatory requirement. Every step, every component, and every test result needs to be recorded so that if something ever goes wrong, it can be traced back and understood. This level of detail is what builds confidence in the safety and reliability of medical devices.
Advanced Manufacturing Techniques for Medical PCBs
When it comes to medical devices, the way a printed circuit board (PCB) is actually made matters a whole lot. It’s not just about getting the components on there; it’s about how precisely and reliably they’re placed and connected. This is where advanced manufacturing techniques really shine, making sure these boards can handle the tough jobs they’re designed for.
Surface Mount Technology (SMT) Precision
Surface Mount Technology, or SMT, is pretty much the go-to for placing tiny components onto a PCB. Think of it like a super-accurate robot arm picking up minuscule parts and setting them down exactly where they need to go. Automated pick-and-place machines can achieve placement accuracy down to 0.01 mm, which is incredibly important for the dense designs we see in modern medical gadgets.
This method is fantastic for fitting a lot of functionality into a small space, like in hearing aids or wearable sensors. The process usually involves printing solder paste, placing components, and then reflow soldering to create the connections. For sensitive medical applications, using nitrogen reflow can help prevent oxidation and ensure a cleaner, more reliable solder joint.
Through-Hole Technology (THT) Durability
While SMT is great for small parts, Through-Hole Technology (THT) is still super important, especially for components that need a bit more mechanical strength. These are the parts with leads that go through holes in the PCB and are then soldered. THT is often used for larger connectors or components that experience more physical stress. It’s a more robust connection method, which is why it’s still favored in certain diagnostic equipment or larger medical systems where durability is key. The assembly typically involves inserting components, followed by wave soldering or selective soldering for more delicate boards. This method provides a strong, reliable connection that can withstand more physical strain.
High-Density Interconnect (HDI) Capabilities
High-Density Interconnect, or HDI, is a real game-changer for medical PCBs. It’s all about packing more circuitry into a smaller area. HDI uses techniques like laser drilling to create microvias, which are tiny holes that connect different layers of the PCB. These vias can be as small as 0.1 mm, allowing for much finer traces and more component placement.
This is absolutely critical for creating the next generation of compact medical devices, from implantable sensors to advanced imaging equipment. HDI also helps improve signal integrity, which is vital for accurate data transmission in devices like ECG monitors. The ability to create these complex, multi-layered boards means we can pack more power and functionality into smaller, more patient-friendly devices. It’s a complex process, but the results are boards that are both smaller and more capable.
The precision involved in these manufacturing techniques directly impacts the reliability and safety of medical devices. Every step, from component placement to soldering, must be executed with extreme care to prevent failures that could have serious consequences for patient care. This is why partnering with manufacturers who specialize in these advanced methods is so important for medical product development.
Here’s a quick look at how these techniques contribute:
- SMT: Ideal for miniaturization and high component density.
- THT: Provides robust connections for mechanically stressed components.
- HDI: Enables smaller form factors and improved signal performance.
These advanced manufacturing processes are the backbone of creating the reliable and sophisticated medical PCBs needed for healthcare in 2025 and beyond.
Rigorous Quality Control and Testing Protocols
When it comes to medical devices, there’s really no room for error. The components inside these machines, especially the printed circuit boards (PCBs), have to work perfectly every single time. That’s why quality control and testing aren’t just afterthoughts; they’re built into the whole process from the start. It’s all about making sure the final product is safe for patients and reliable for healthcare professionals.
Automated Optical Inspection (AOI) and X-Ray
After the components are placed on the board, we use Automated Optical Inspection (AOI) to give everything a good look. This system uses cameras to check for things like misplaced parts, incorrect orientation, or solder joint issues. It’s pretty fast and catches a lot of common problems. For components that have hidden connections, like BGAs (Ball Grid Arrays), we bring in X-ray inspection. This lets us see inside those tricky spots to make sure the solder connections are solid and there are no voids. This dual approach catches a wide range of potential defects before they become bigger problems.
Functional and In-Circuit Testing (ICT)
Once the board is assembled, we move on to testing its actual performance. In-Circuit Testing (ICT) is like a detailed check-up for the board. It tests individual components and connections to make sure they’re all working as they should. Following that, we perform functional testing. This is where we simulate real-world operating conditions to see if the board performs its intended tasks correctly. It’s a critical step to confirm the board does what it’s supposed to do in the device it’s designed for. We’re talking about making sure signals are clean and the board responds as expected under various loads.
Environmental Stress Screening and Validation
Medical devices often operate in challenging environments, and they need to withstand more than just normal use. Environmental Stress Screening (ESS) puts the assembled PCBs through a series of rigorous tests designed to simulate these conditions. This can include temperature cycling, where the board is repeatedly heated and cooled, and humidity exposure. These tests help us identify any weaknesses or potential failure points that might not show up during standard testing. It’s about pushing the board a bit to see how it holds up, which gives us confidence in its long-term durability. We also conduct validation to confirm that the manufacturing processes themselves consistently produce boards that meet all specifications.
The entire testing and validation process for medical PCBs is meticulously documented. Every test result, every parameter, and every piece of equipment used is recorded. This creates a clear trail, showing exactly how the board was made and tested. This level of detail is absolutely necessary for regulatory bodies and for ensuring accountability throughout the product’s lifecycle. It’s not just about passing tests; it’s about proving it through records.
We work with partners who understand these demands, like ANZER USA, to ensure our medical PCB assemblies meet the highest standards for safety and reliability.
Navigating Regulatory Compliance in Medical PCB Assembly
When you’re building electronics for the medical field, it’s not just about making things work; it’s about making them work safely and reliably, every single time. This means jumping through a lot of regulatory hoops. It can feel like a maze, but getting it right is non-negotiable because patient well-being is on the line.
Adhering to ISO and FDA Standards
Two big players here are the International Organization for Standardization (ISO) and the U.S. Food and Drug Administration (FDA). ISO sets the rules for quality management systems, and for medical devices, ISO 13485:2016 is the standard you really need to pay attention to. It’s like the gold standard for making sure your manufacturing processes are solid and consistent. Then there’s the FDA, which has its own set of requirements, especially if your device will be used in the United States.
They look closely at documentation, how you control your designs, and how you validate everything. It’s a lot to keep track of, but manufacturers who have a good handle on these standards are usually the ones who succeed in the medical market. You can find a lot of helpful information on PCB assembly components.
Method Validation and Facility Approval
It’s not enough to just have the right paperwork; you have to prove your processes work. This is where method validation comes in. You need to show that the way you build your PCBs is accurate, safe, and repeatable. This involves checking everything from the environmental conditions in your factory to the specific settings on your machines at each step of production. Think of it like a chef meticulously following a recipe and documenting every temperature and time. Facility approval is also a big deal. Your manufacturing site needs to meet strict conditions and often undergoes regular inspections to make sure it’s up to snuff for medical device production.
Ensuring Traceability and Documentation
In the medical world, you absolutely have to know where every single part came from and what happened to it along the way. This is called traceability. It means keeping detailed records of every component used, every step of the assembly process, and every test performed. If something ever goes wrong, you need to be able to trace it back to its source. This meticulous documentation isn’t just for your own records; it’s often required by regulatory bodies. It builds trust and shows that you’re serious about quality and safety. Without it, getting your medical device to market can be a real uphill battle.
Addressing Challenges in Medical PCB Solutions

Building PCBs for medical gear isn’t always straightforward. There are some tricky bits to work through, but thankfully, there are ways to handle them. It’s all about being prepared and knowing what to expect.
Overcoming Miniaturization Demands
Medical devices are getting smaller, and that means the circuit boards inside have to shrink too, without losing any of their smarts. This is where High-Density Interconnect (HDI) technology really shines. It lets us cram more tiny wires and connections into a smaller space. Think trace widths as small as 2 mils – that’s super fine! But, making these super-compact boards is more complex. You really need a manufacturing partner who’s got the high-tech gear and knows their way around these intricate designs. It’s not something you can just wing.
Ensuring Supply Chain Reliability
When you’re making medical equipment, you can’t afford delays. Waiting around for parts when lives are on the line is just not an option. This is why having a solid supply chain is so important. Working with a company that has good relationships with component suppliers can make a huge difference. They often buy parts in larger quantities, which can help avoid those frustrating shortages. Some even keep stock of critical components, so they’re ready when you need them.
Managing Part Limitations and Material Selection
Sometimes, the parts you need just aren’t available in the right size or with the right specs for your super-small medical device. Or, certain materials, like some metals, might react badly with other things in the device, which is a no-go for medical applications. Plus, you’ve got to think about electromagnetic interference (EMI) – you don’t want your device messing with other electronics or vice-versa. Choosing the right materials and components that are both reliable and safe for medical use is a big part of the puzzle. It often means careful planning and sometimes finding clever workarounds.
The pressure to make medical devices smaller, more powerful, and more reliable is constant. This means PCB manufacturers have to be really smart about how they design and build these boards, paying close attention to every tiny detail from the materials used to how the components are placed. It’s a balancing act between advanced technology and practical, safe manufacturing.
Future Trends Shaping Medical PCB Assembly
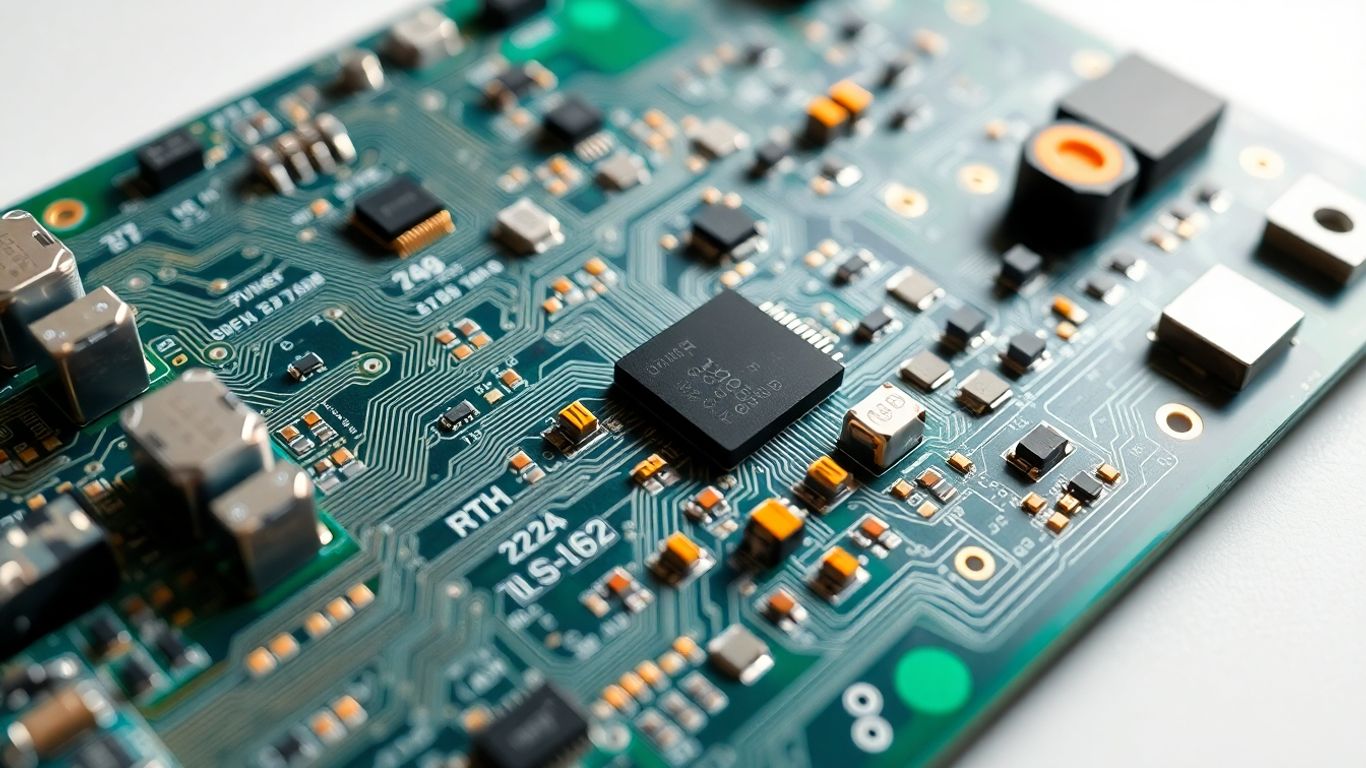
The world of medical electronics is always changing, and the printed circuit boards inside them are changing right along with it. It’s pretty exciting to see what’s coming next, especially when it comes to making devices smaller, smarter, and more connected. We’re seeing some big shifts that are going to make a real difference in patient care and how we monitor our health.
The Rise of Flexible and Wearable PCBs
One of the biggest things happening is the move towards flexible and wearable PCBs. Think about those fitness trackers or continuous glucose monitors you see everywhere now. They need circuit boards that can bend and move with the body without breaking. These flexible boards often use special materials like polyimide, and they can be incredibly thin, sometimes just 0.1 mm. This allows for much more comfortable and discreet health monitoring devices that can be worn for extended periods. It’s a game-changer for remote patient monitoring and personal health management.
Integrating IoT and AI Capabilities
Another huge trend is how medical devices are becoming connected, thanks to the Internet of Things (IoT). This means PCBs need to have built-in wireless features to send and receive data. High-Density Interconnect (HDI) designs are really important here because they allow for very tight layouts, which is perfect for fitting in all the tiny chips and antennas needed for wireless communication. On top of that, artificial intelligence (AI) is starting to play a bigger role.
Medical devices are using AI for things like diagnostics, which means the PCBs need to handle much faster data speeds, sometimes over 5 Gbps. This pushes the limits of how we design circuit boards to maintain signal integrity and manage complex layers. It’s all about making devices smarter and more capable of processing information right where it’s needed.
Advancements in High-Speed Signal Transmission
As devices get more complex and data needs increase, the ability for PCBs to handle high-speed signals reliably becomes super important. This isn’t just about speed; it’s about making sure the data gets through accurately, even in challenging environments. Manufacturers are constantly working on new ways to design and build PCBs that can support these demanding signal requirements. This often involves using advanced materials and manufacturing techniques to minimize signal loss and interference.
For anyone looking to build cutting-edge medical devices, working with a manufacturer that understands these high-speed transmission needs is key. Companies like ANZER, with their extensive experience and certifications, can be a great partner in this area, offering solutions from prototypes to full production runs for complex electronic assemblies.
The push for smaller, more integrated, and smarter medical devices means that PCB technology has to keep up. This involves not just shrinking components but also improving how signals travel and how devices communicate with each other and with healthcare providers. It’s a complex but vital area of development for the future of medicine.
Wrapping It Up: Your Next Steps for Medical PCBs
So, we’ve talked a lot about what goes into making medical PCBs – the tricky bits like making them small, keeping them super reliable, and following all the rules. It’s not exactly simple stuff. But the good news is, there are companies out there that really know their stuff. Partnering with the right folks means you get the benefit of their experience and fancy equipment, which can save you headaches and maybe even some cash.
As technology keeps moving forward, especially with things like wearable tech and smarter devices, the need for these specialized PCBs will only grow. It’s all about making sure the devices that help people stay healthy and safe are built right, from the very first circuit board.
Frequently Asked Questions
Why are special circuit boards needed for medical devices?
Medical devices, like heart monitors or X-ray machines, need to be super reliable because people’s health depends on them. Special circuit boards, called medical PCBs, are built with extra care and tough testing to make sure they work perfectly every time and don’t fail, even in tricky situations. This helps keep patients safe.
What are the main ways medical circuit boards are put together?
There are two main methods. One is called Surface Mount Technology (SMT), where tiny parts are stuck right onto the board’s surface. This is great for making devices really small, like smartwatches that track your health. The other is Through-Hole Technology (THT), where parts with little wires go through holes in the board and are soldered. This makes a stronger connection and is often used for bigger, tougher parts in machines.
How do companies make sure medical circuit boards are good quality?
Companies use lots of checks! They have machines that use cameras to look for any tiny mistakes on the board, like parts put on wrong or bad solder joints. They also test the boards to see if they work like they’re supposed to and can handle tough conditions, like hot or cold temperatures. This is all to make sure the boards are super dependable.
Are there special rules for making medical circuit boards?
Yes, definitely! Because medical devices are so important, there are strict rules and standards, like those from the FDA and ISO. Companies have to follow these rules very closely during design and making. They also need to keep detailed records of everything they do, so if there’s ever a question, they can show exactly how the board was made and tested.
What’s hard about making circuit boards for new medical gadgets?
One big challenge is making the boards smaller and smaller to fit into tiny devices, but still making them work just as well. Another is making sure they can be made reliably, even if there’s a shortage of certain parts. Choosing the right materials that won’t cause problems is also tricky.
What’s new and exciting coming for medical circuit boards?
We’re seeing more flexible boards that can bend, which are perfect for wearable health trackers. Also, more devices will be able to connect to the internet (IoT) to send health data. Soon, some boards might even help devices use artificial intelligence (AI) to help doctors figure out what’s wrong with patients faster.
At ANZER, we’ve spent over three decades building a legacy of excellence in PCB assembly, contract electronics manufacturing, and electronics services. Our commitment to quality and customer satisfaction has made us a trusted name in the industry.
What started as a mission to create top-quality electronics has grown into a passion for empowering businesses with innovative solutions. Over the years, we’ve refined our approach to be a flexible and dependable partner for PCB assembly.
Today, ANZER is recognized as a leader in the field, driven by cutting-edge technology, a spirit of innovation, and an unwavering dedication to exceeding customer expectations.

